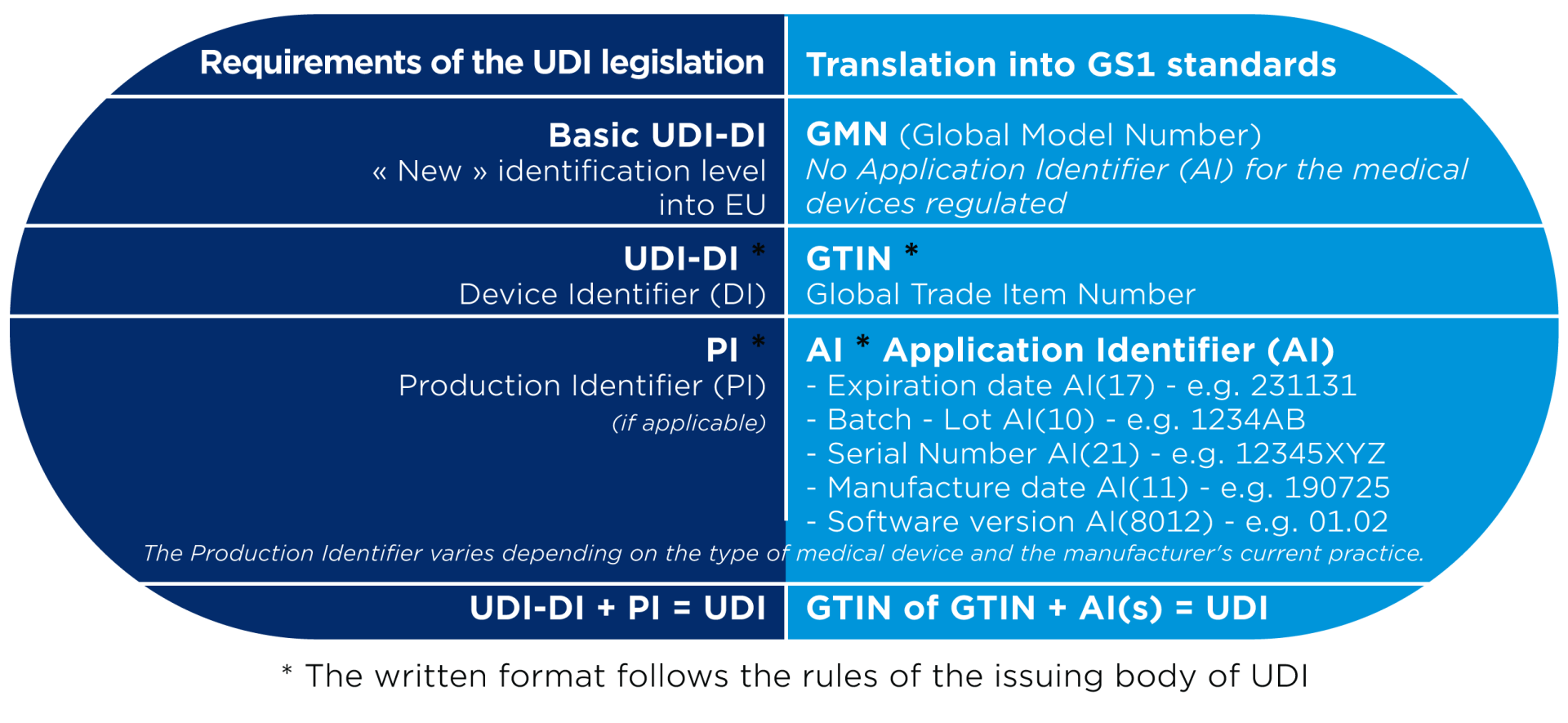
A UDI (Unique Device Identifier) is a code that is unique in the world for identifying medical devices. The code consists of two parts:
- UDI-DI (Device Identifier or DI): the fixed part of the code that uniquely identifies each medical device via a GTIN.
- UDI-PI (Production Identifier or PI): the variable part that adds production information, such as batch number, serial number or expiry date.
International regulations, such as the MDR and IVDR in Europe and FDA guidelines in the United States, require suppliers to assign a UDI to all medical devices.
GS1 is an accredited UDI-issuing organisation that helps you comply with international regulations, such as EU MDR & IVDR and US FDA, that require suppliers to assign a UDI to their products.
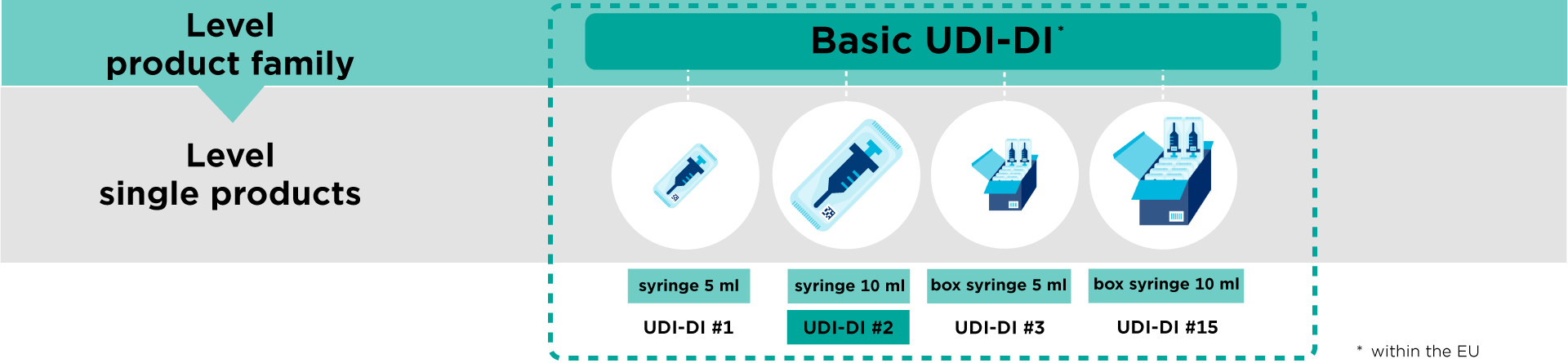
In Europe, the UDI-DI is also used to create the Basic UDI-DI.
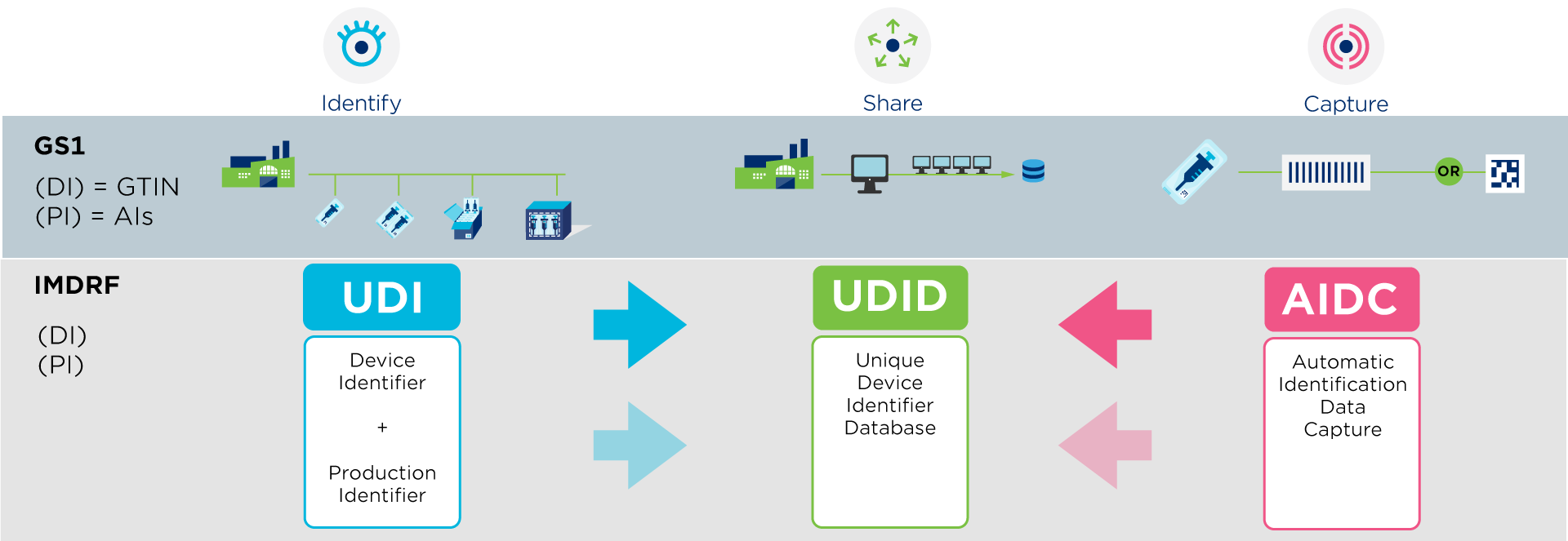
Compliance with UDI requirements for medical devices is based on three principles, as defined by the International Medical Device Regulators Forum (IMDRF). These global guidelines form the basis for national legislation, allowing each region to meet its specific requirements.
- Identification of medical devices by means of a UDI.
- The ability to scan information via a barcode. As such, GS1 DataMatrix and GS1-128 barcodes are permitted in the European Union and contain all legally required information in a single barcode.
- Sharing product information in a regulatory database, such as EUDAMED for the European Union or GUDID in the United States.