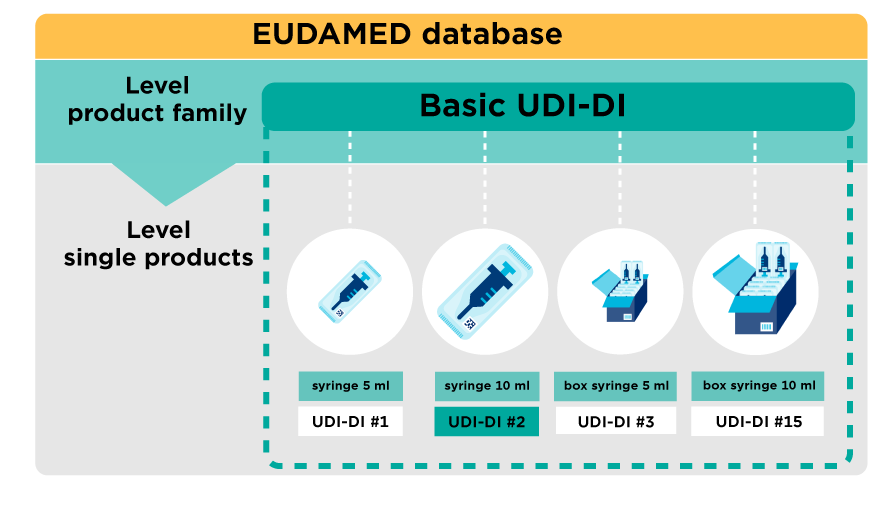
As a manufacturer of medical devices, you have already assigned Unique Device Identifiers (UDI-DI) to your products. For the Medical Device Regulation (MDR) and In-Vitro Diagnostic Regulation (IVDR), these products now also need to be registered in EUDAMED using Basic UDI-DIs. This can be done easily with My Basic UDI-DI Manager.
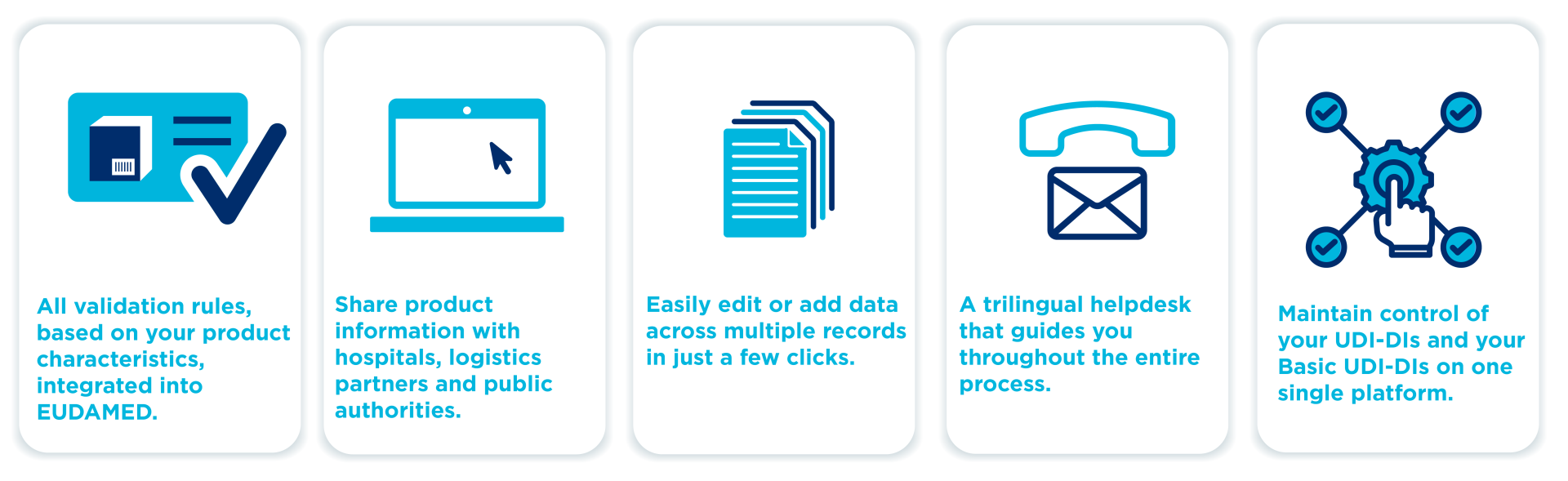
My Basic UDI-DI Manager is GS1 Belgium & Luxembourg's solution to:
- easily create and manage your Basic UDI-DI or Global Model Number (GMN);
- filling in the data required by EUDAMED based on the product properties;
- linking the unique identification numbers of your medical device UDI-DI (or GTIN) to your Basic UDI-DI or GMN;
- simply publishing the information to EUDAMED with integrated feedback from EUDAMED.
To use My Basic UDI-DI Manager, you need a paid subscription to My Product Manager Share and My Basic UDI-DI Manager.
View pricing Request acces to My Basic UDI-DI Manager Follow a free webinar