EUDAMED as a gamechanger: how data became an asset at Ergotrics
At Ergotrics, the team faced a challenge: to comply with the MDR (Medical Device Regulation), the Basic UDI-DIs of their medical devices had to be registered in EUDAMED. Not an easy task, but also an opportunity to take a closer look at how product data was managed. With the help of My Basic UDI-DI Manager and the support of GS1, the team decided to bring all their scattered information together in one central tool. Managing Director Inge Bruynooghe explains how this obligation became a smart step towards more efficient working.
Who is Ergotrics and what exactly do they do?
Ergotrics is a Belgian start-up that developed an ergonomic solution to move patients in an operating room or intensive care unit. Thanks to compressed air in the inflatable Ergotrics cushions, a patient can be moved easily so that healthcare staff no longer have to do this manually. The nurse places the cushion in the correct position and inflates it until the patient is positioned properly.

CEO

Although their products belong to the lowest MDR risk class, they still need to complete several procedures (ISO certification, traceability, EUDAMED…) to comply with the legislation. Registration in EUDAMED is one of these steps – and My Basic UDI-DI Manager answers several questions about data exchange.
“We do not use EUDAMED every day, so support from GS1 for the publications was really welcome,” says Inge. “Publishing in EUDAMED is official, and it is difficult to change data afterwards. Some fields can no longer be edited once they are published. That’s why it is useful to first run validations in My Basic UDI-DI Manager. Jonathan Van Herpe, account manager for healthcare, was also very available. An online meeting was scheduled quickly, instead of sending endless emails with different people replying,” she adds.
Product information in different shapes and formats
Ergotrics was already a GS1 Belgilux member for their company prefix. “But we were not yet sharing our product information in My Product Manager,” explains Inge. “We had a good internal documentation system for technical files, needed for ISO certification. But these files were in Word, PDF or Excel, so not stored in a structured way.”
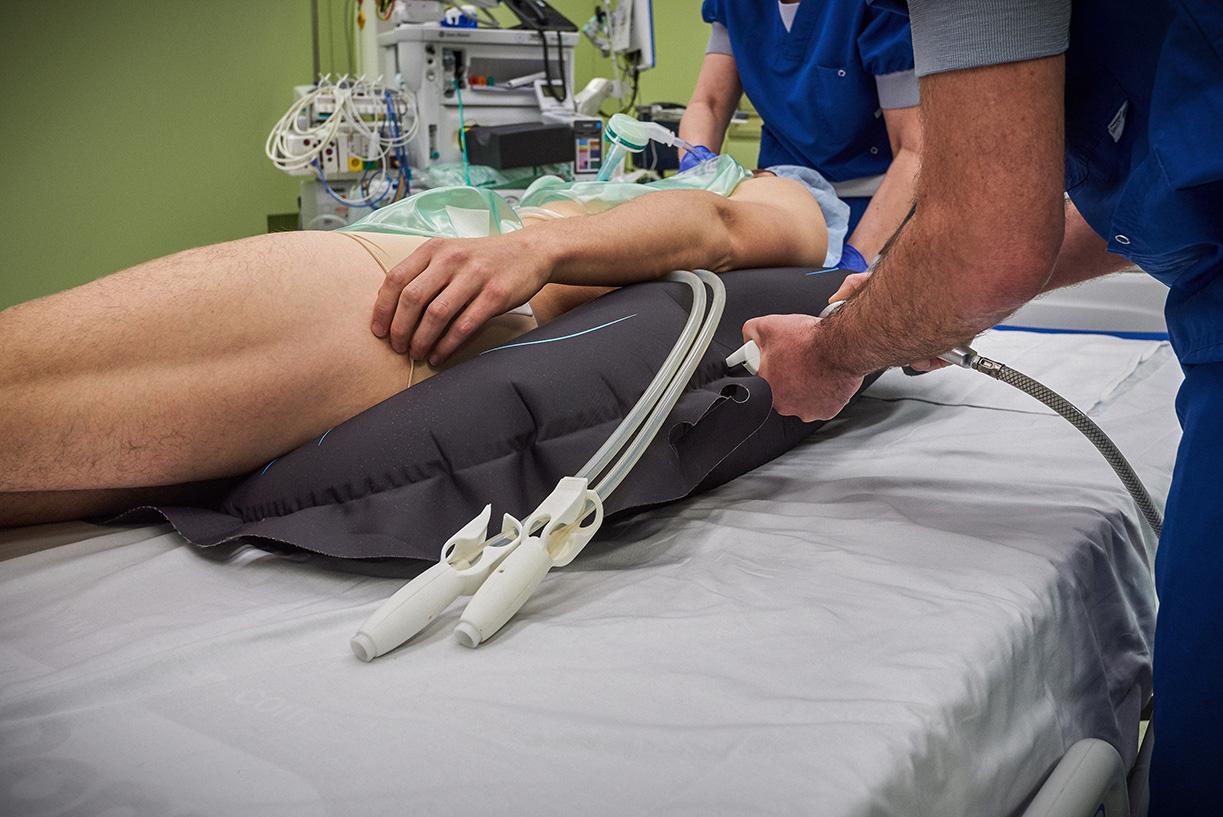
The EUDAMED registration was the push they needed to centralise everything. “We turned this obligation into an opportunity and added not only the basic EUDAMED information, but also the logistical data to My Product Manager,” says Inge. “This was quite easy for our assortment, because you can add or update information per product group.”
Good product information means simpler processes
Now that all product information is stored centrally in My Product Manager, several processes have become simpler. For ISO certification, product data must be checked regularly. Thanks to My Product Manager, this can now be exported with one click.
“When customers ask for specific lists, we refer them to GS1,” says Inge. “We hope the sector will also use this as a logistics tool. This way, we no longer have to fill in their own Excel sheets or Word documents for each customer,” Inge concludes. This will only make the healthcare sector more efficient.